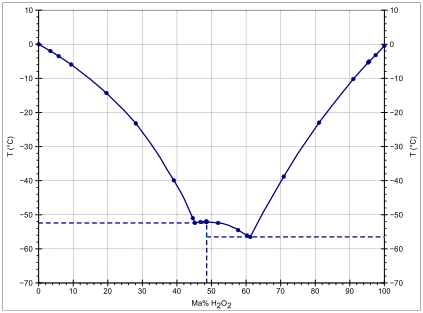
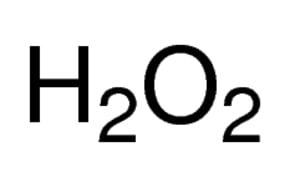Table 1 from Biodecomposition of Hydrogen Peroxide (H 2 O 2 ) in Water and in Organic Solvents Using Saccharomyces cerevisiae Meyen ex E.C. Hansen (Fungi: Ascomycota) | Semantic Scholar

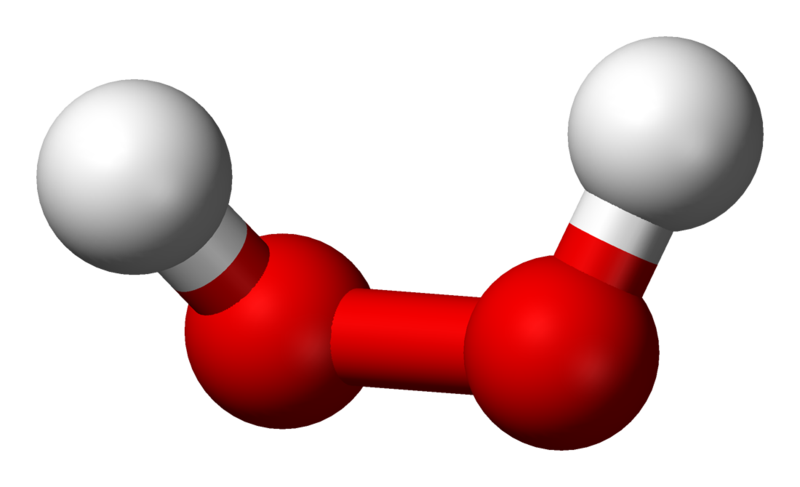
A solution of hydrogen peroxide is 15.2% by mass. What is the molarity of the solution? Assume that - Brainly.in

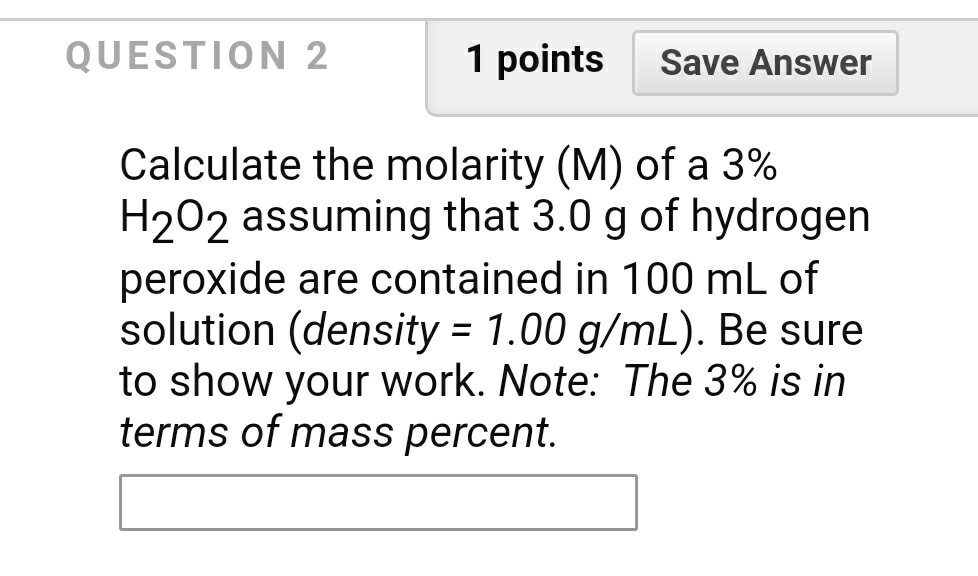
OneClass: If I have hydrogen peroxide, molecular weight(g/mol) is 34.02, its density (g/ml) is 1.2, i...
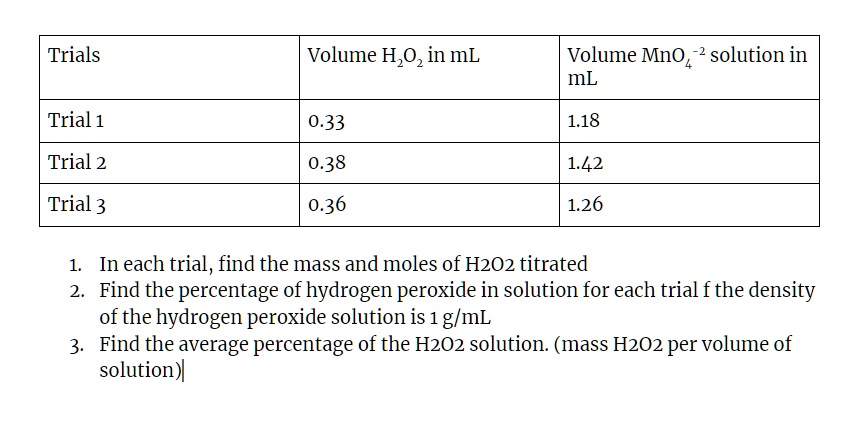
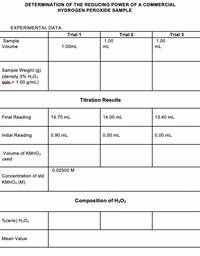
SOLVED: Trials Volume H,Oz in mL Volume Mno solution in mL Trial 1 0.33 Trial 2 0.38 1.42 Trial 3 0.36 1.26 In each trial, find the mass and moles of H2O2

SOLVED: 1,100 kgIm' Whal would you expect the mass ofthat solution to be if it 309 hydrogen peroxide has density of filled container with volume of 0,03 m ? 990 kg 33kg 0000027 kg 36,666.67 kg

OneClass: I am having a difficult time understanding this problem,can someone please assist? Than...
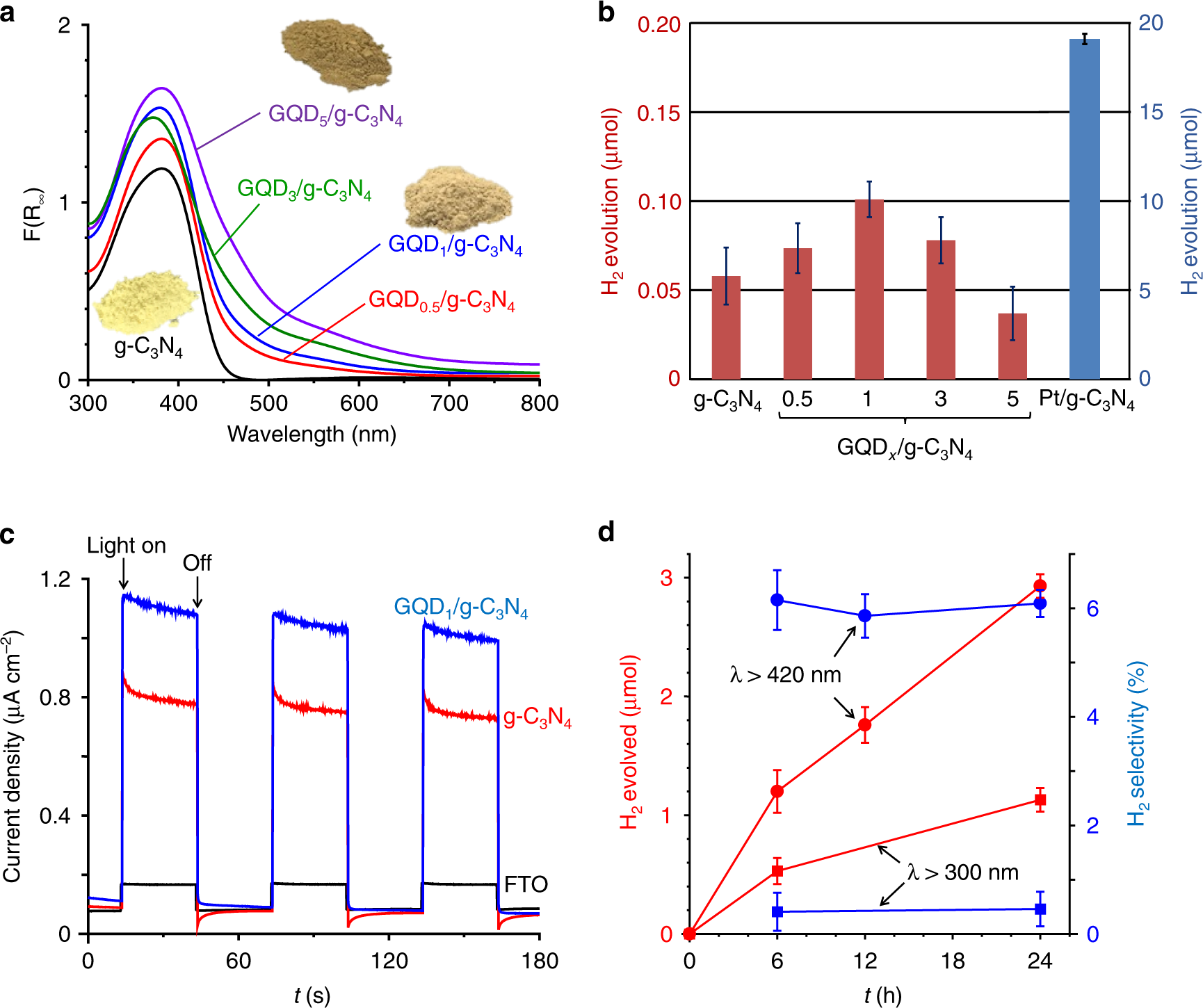
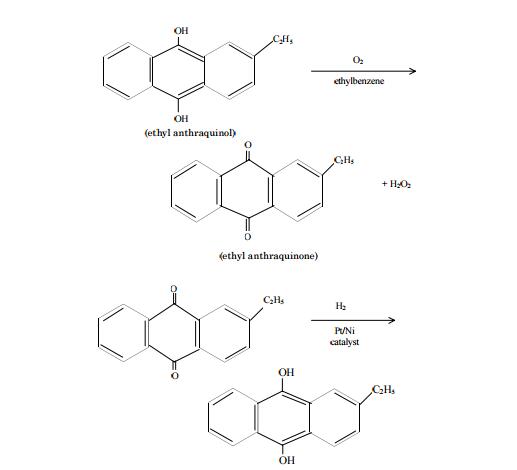
Photocatalytic hydrogen peroxide splitting on metal-free powders assisted by phosphoric acid as a stabilizer | Nature Communications



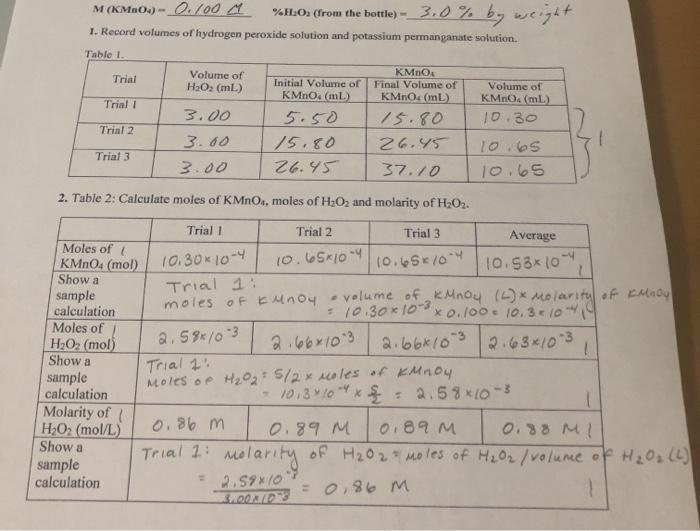
![ANSWERED] An aqueous solution contains 5.0% hydroge... - Physical Chemistry ANSWERED] An aqueous solution contains 5.0% hydroge... - Physical Chemistry](https://media.kunduz.com/media/sug-question/raw/76653404-1659009310.8708293.jpeg?h=512)