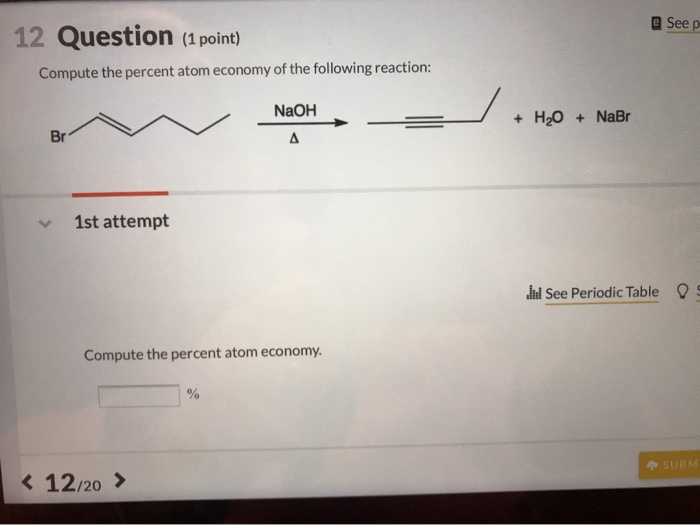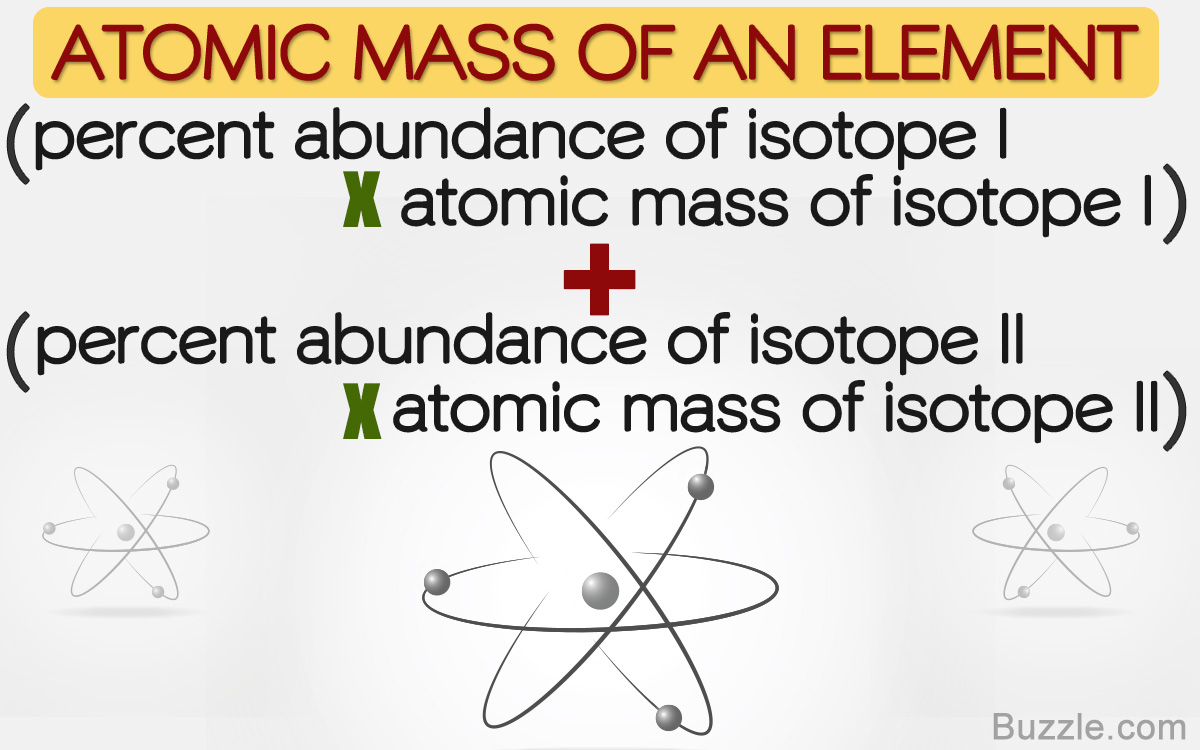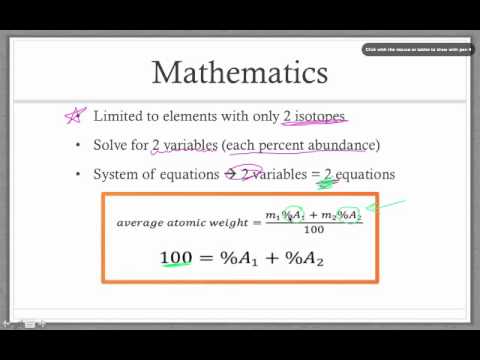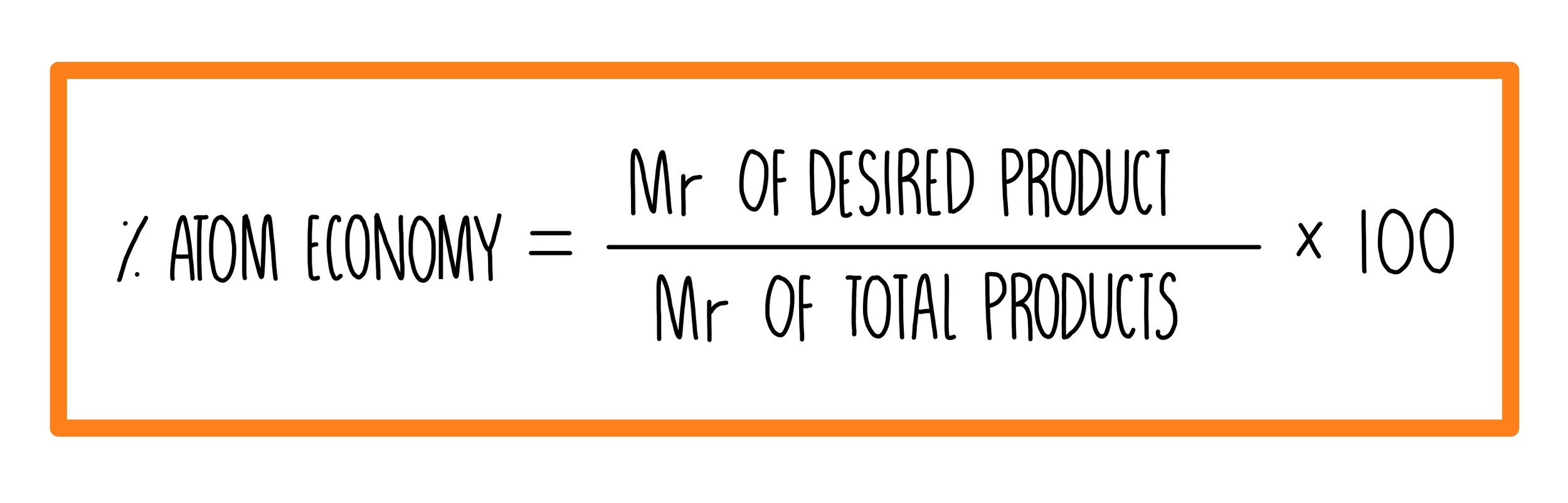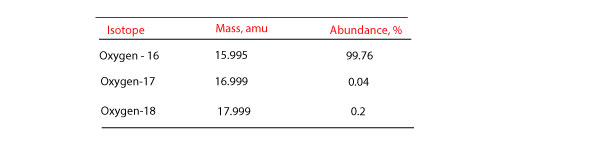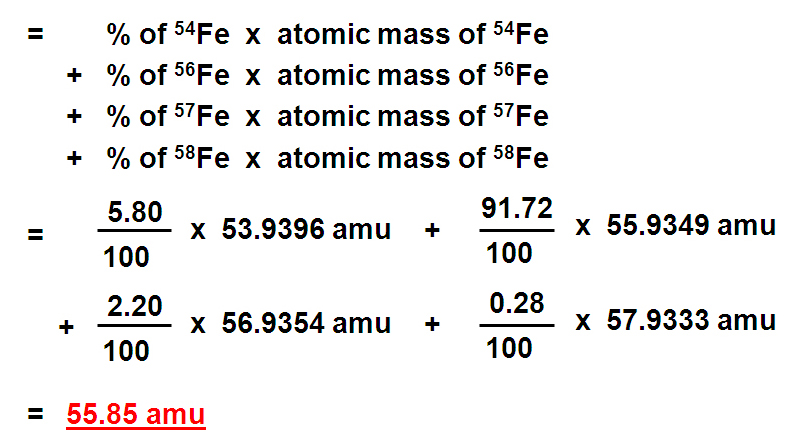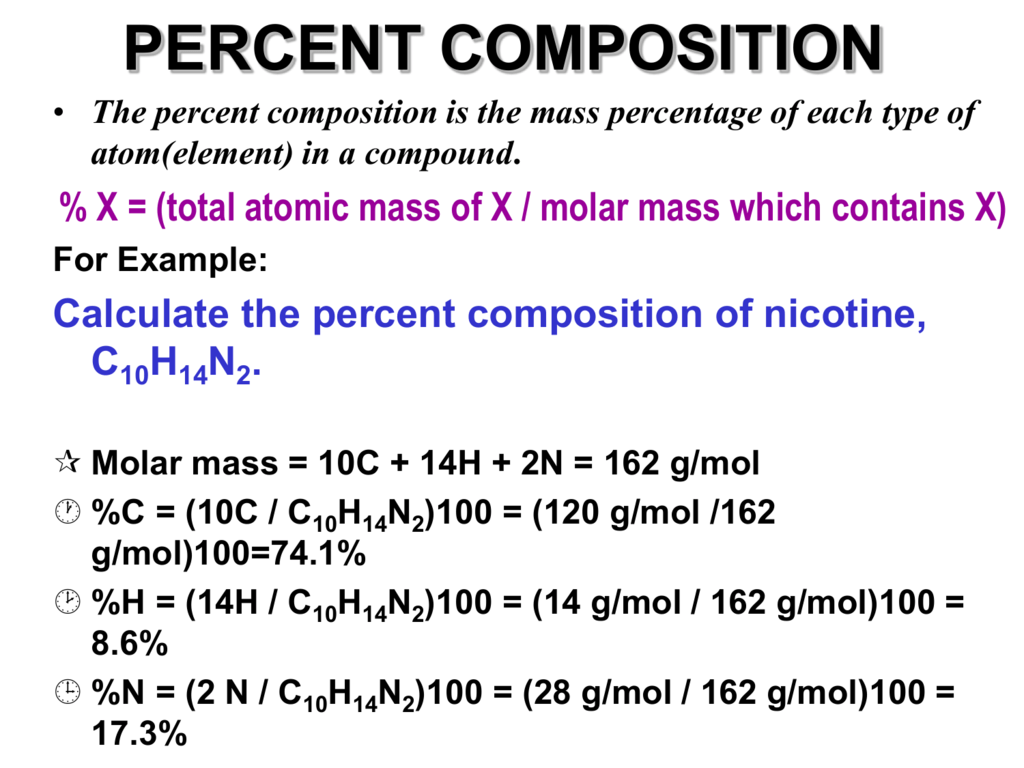
Naturally occurring boron consists of two isotopes whose atomic weight are 10.01 and 11.01 . The atomic weight of the natural boron is 10.81 . Calculate the percentage of each isotopes in natural boron.

Calculating Percent Composition and Determining Empirical Formulas - Video & Lesson Transcript | Study.com