Atomic radius of `Li is 1.23 Å` and ionic radius of `Li^(+)` is `0.76 Å`. Calculate the percentage - YouTube
Derive a formula for radius of the stable orbit of hydrogen atom on the basis of Bohr model. Prove that in hydrogen - Sarthaks eConnect | Largest Online Education Community
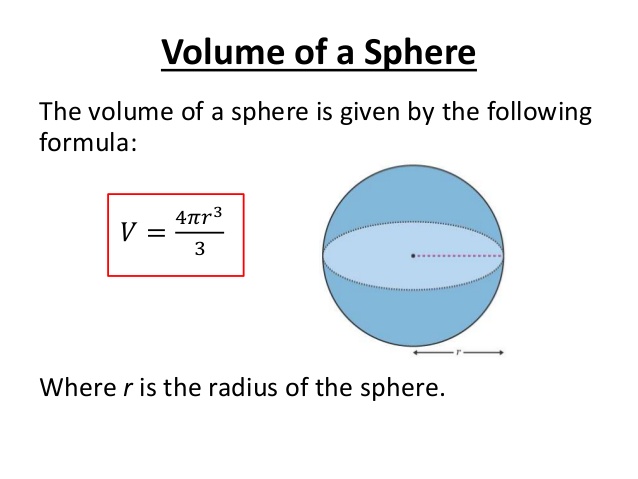
Atomic radius is of order 10^-8 cm and nuclear radius is of order 10^-13. calculate what fraction of atom is occupied by nucleus? | Socratic
Derive a formula for radius of the stable orbit of hydrogen atom on the basis of Bohr model. Prove that in hydrogen - Sarthaks eConnect | Largest Online Education Community
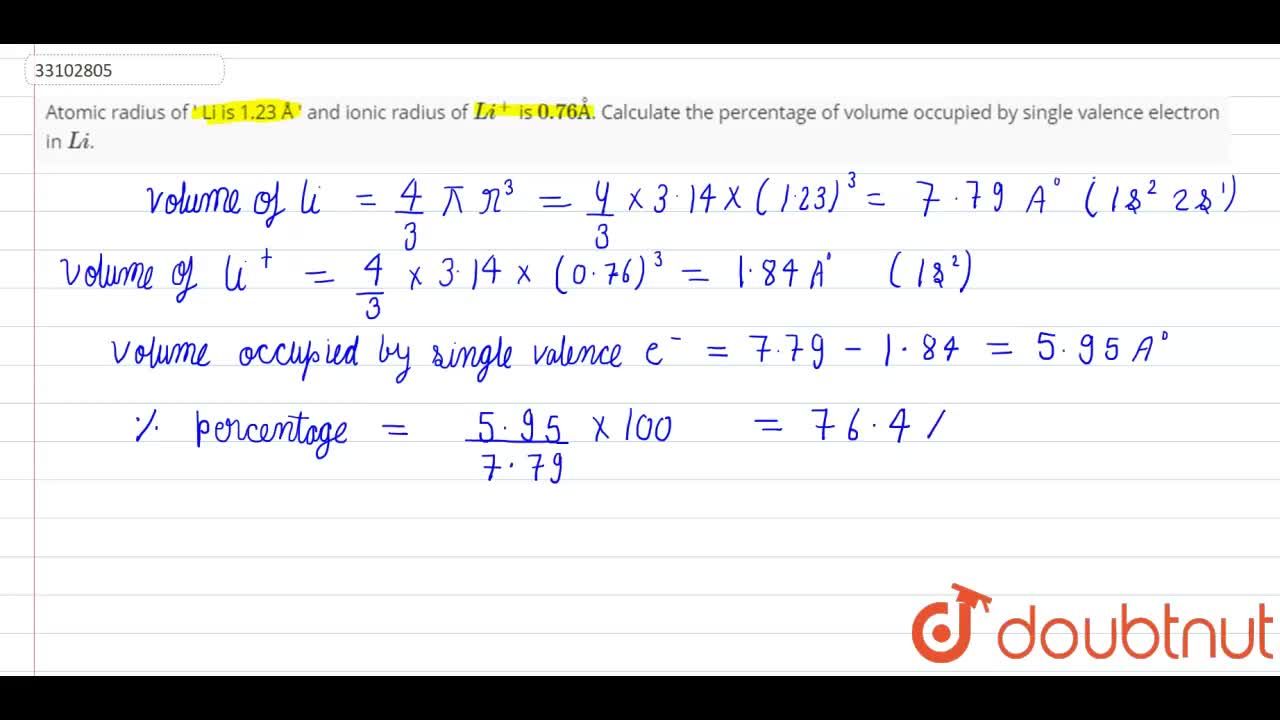
Atomic radius of Li is 1.23 Å and ionic radius of Li^(+) is 0.76 Å. Calculate the percentage of volume occupied by single valence electron in Li.

Welcome to Chem Zipper.com......: Atomic radius is the order of 10^-8 cm. and nuclear radius is the order of 10^--13 cm. Calculate what fraction of atom is occupied by nucleus



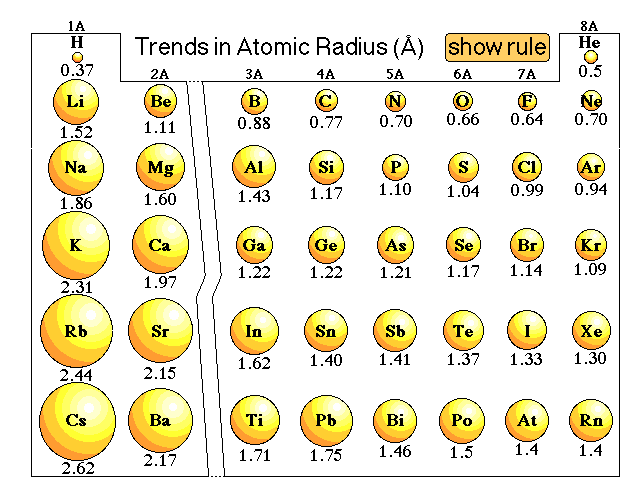


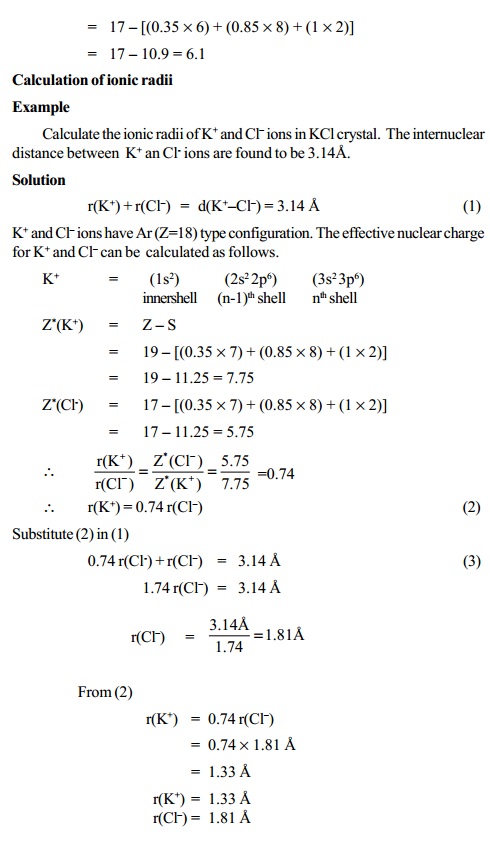
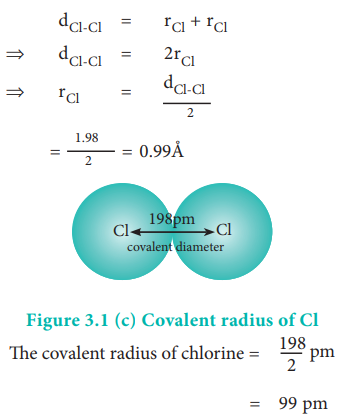
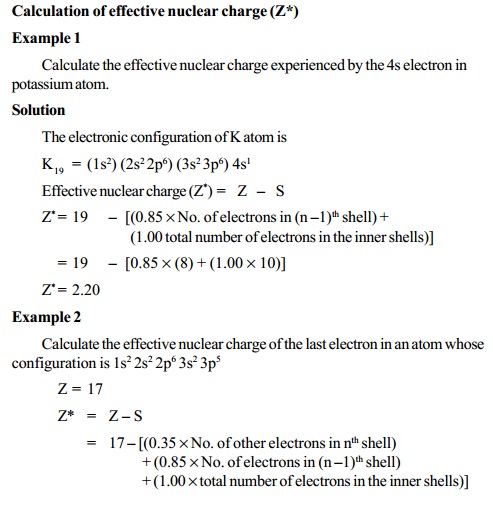







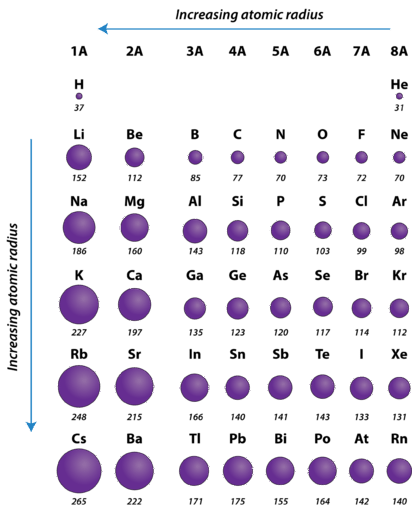
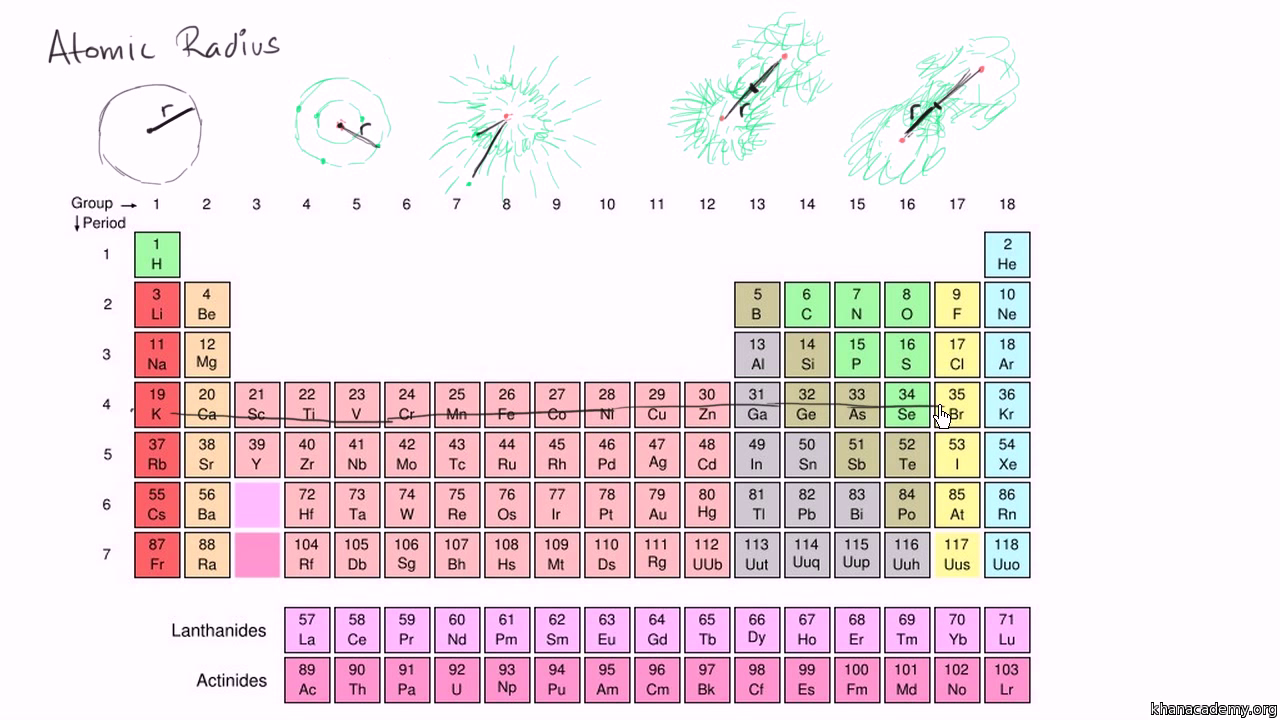
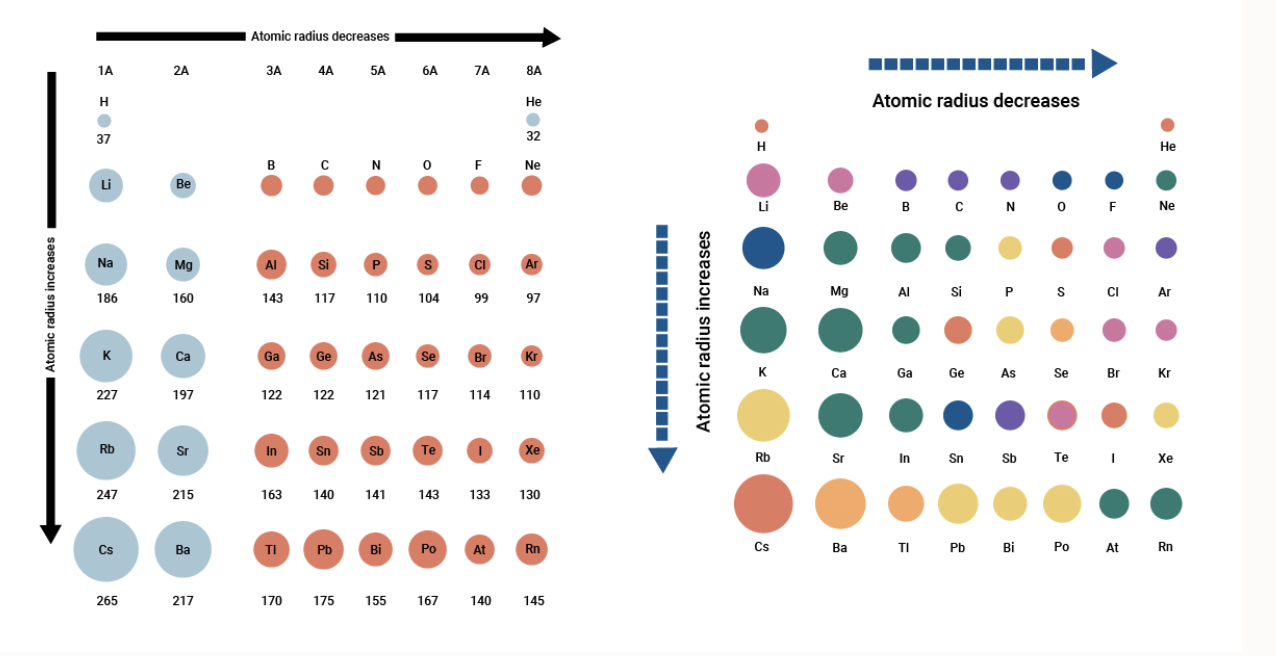
:max_bytes(150000):strip_icc()/PeriodicTable_AtomSizes-56a131193df78cf772684720.png)