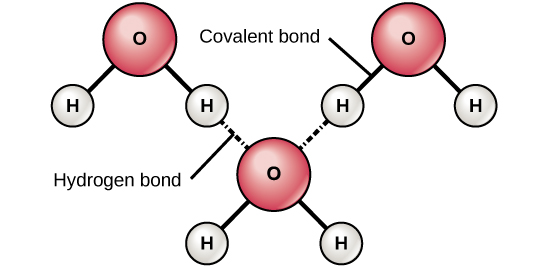
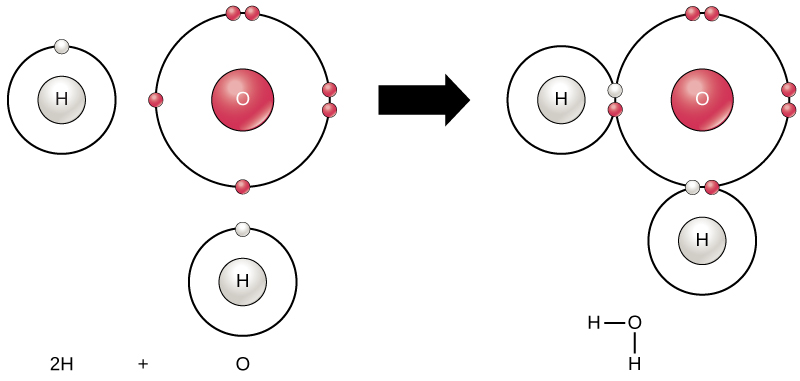
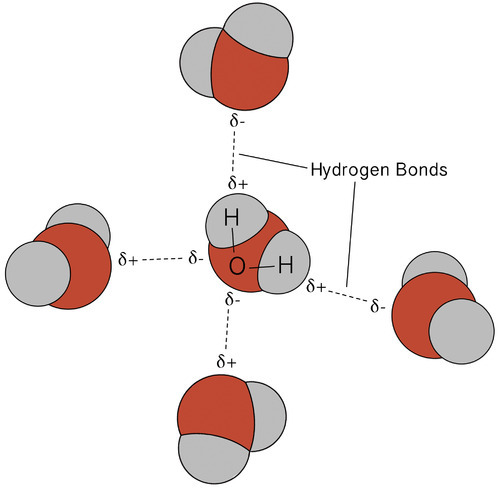
Hydrogen bonding between molecules. A) ) Hydrogen bond formed between... | Download Scientific Diagram
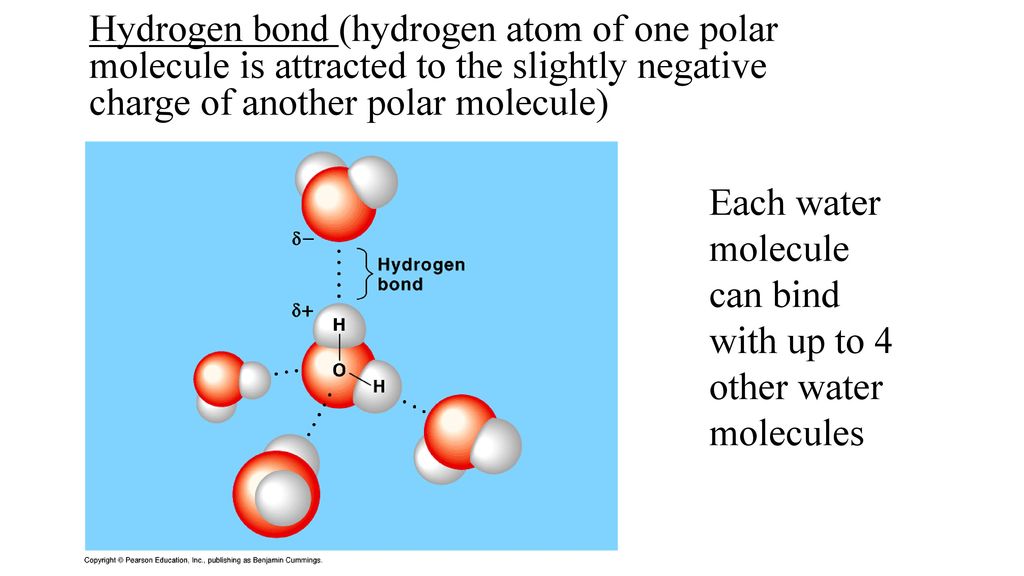
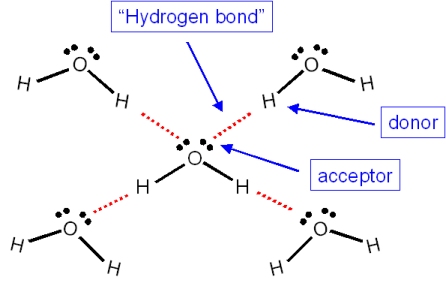
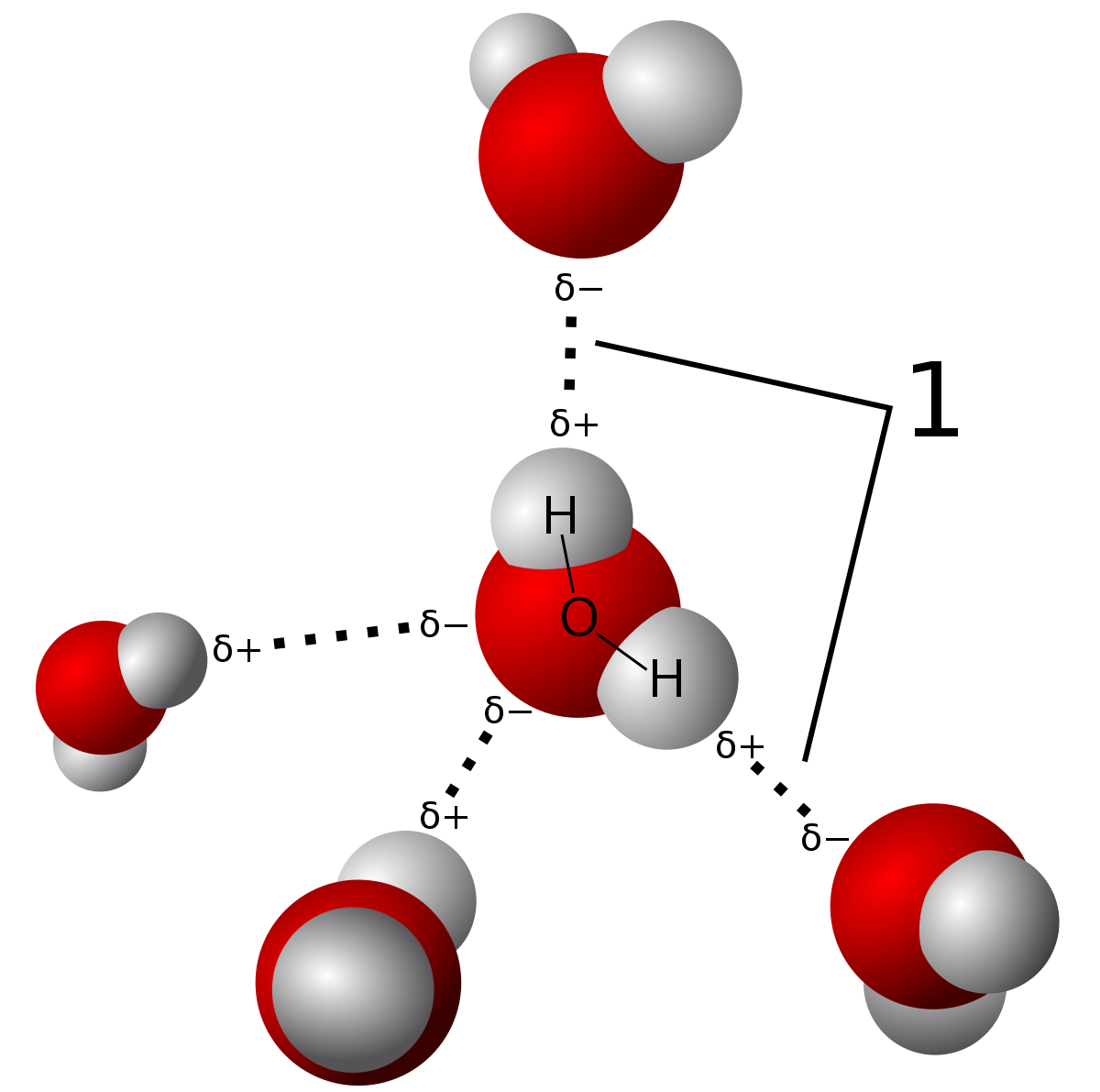
If H2O was linear, would the water molecules still be able to form hydrogen bonds with each other? Why? - Quora

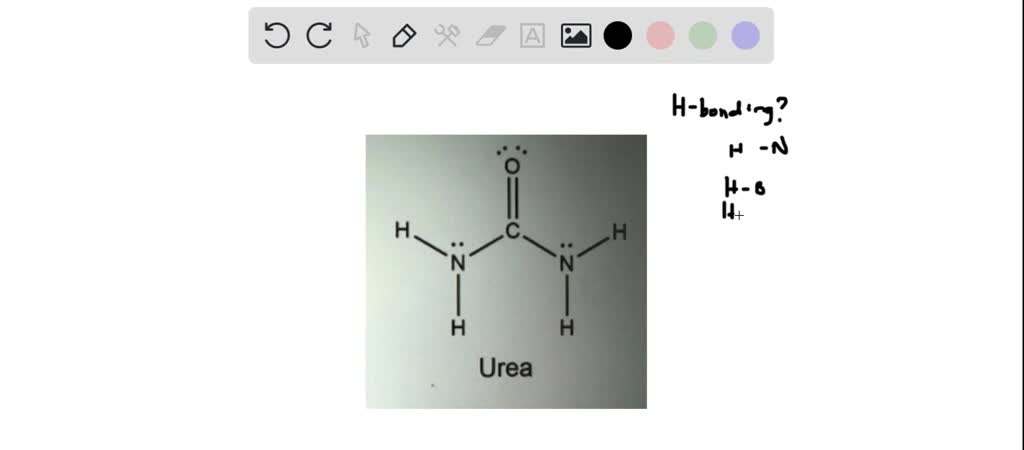
What is the maximum theoretical number of water molecules that one urea molecule can hydrogen bond with? | Homework.Study.com
Both water and methanol have anomalously high boiling points due to hydrogen bonding, but the boiling point of water is greater than that of methanol despite its lower molecular mass. Why? -