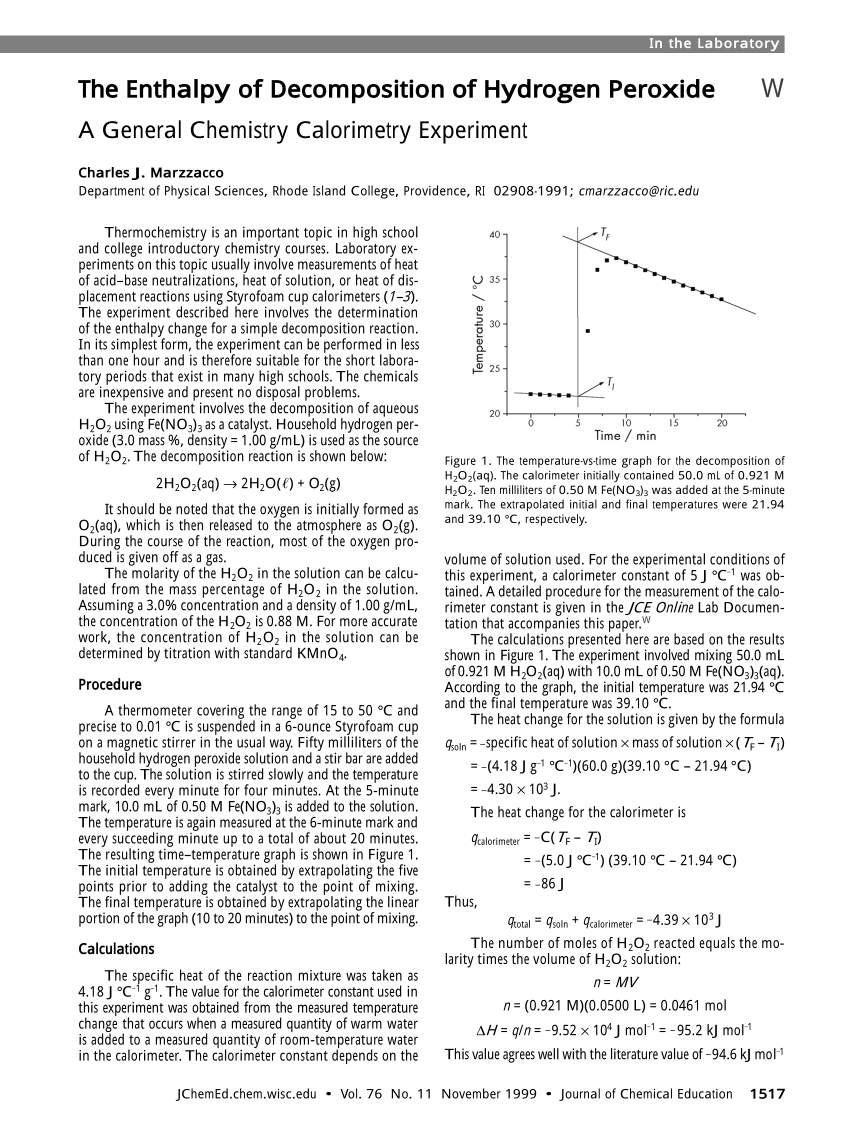
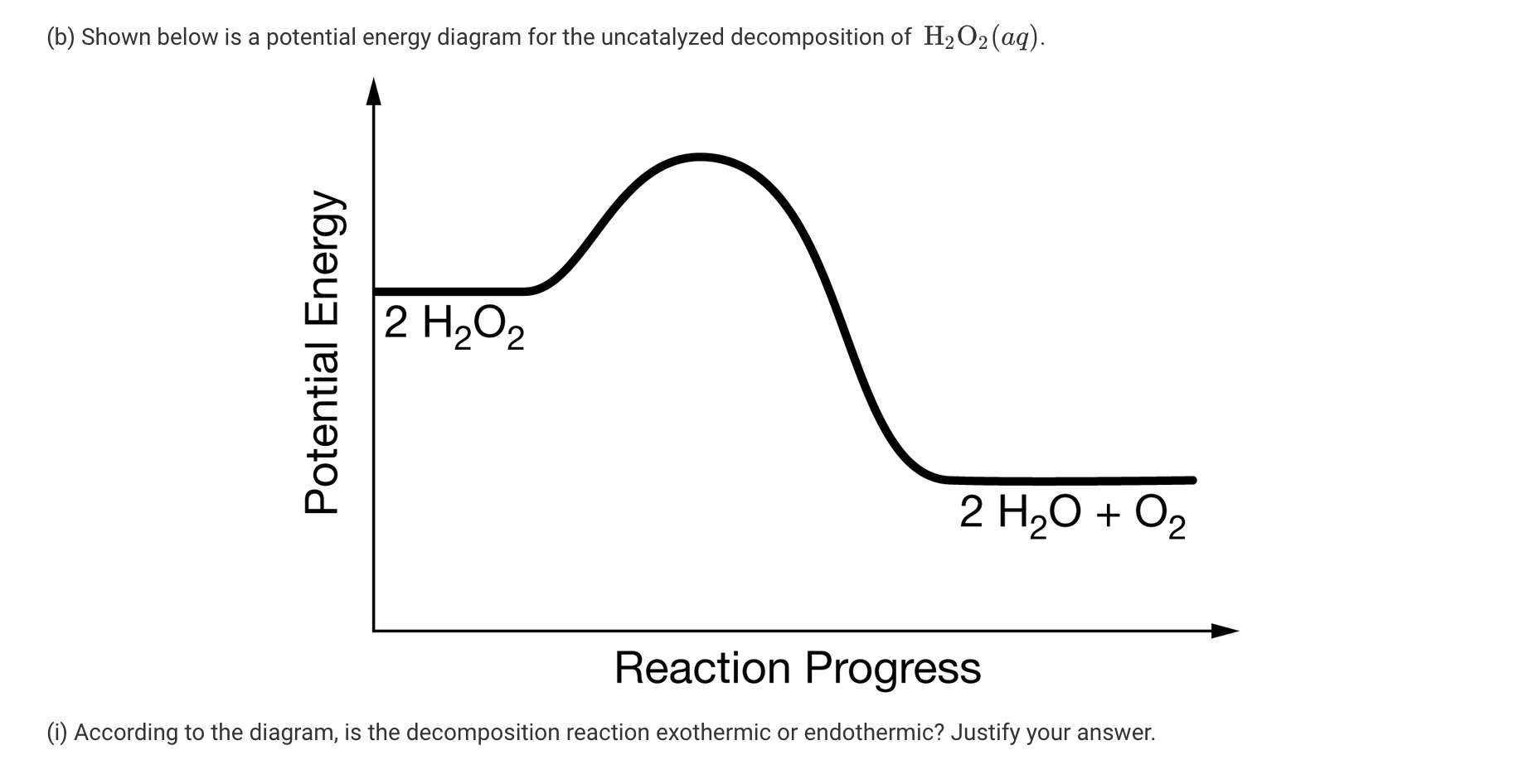
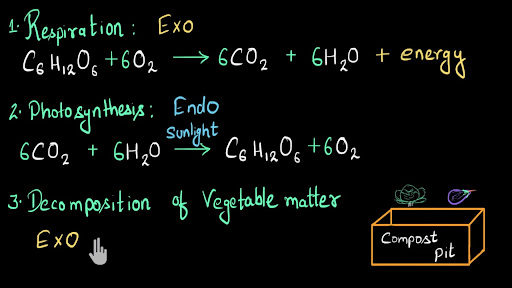SOLVED: If 2 mol of hydrogen peroxide is decomposed, the reaction enthalpy (ΔH) is -54.04 kJ. If 0.814 g of H2O2 decomposes, how much heat (kJ) will be produced? Is this reaction

The enthalpy changes at 298 K in successive breaking of O - H bonds of water are: H2O→ H(g) + OH(g); Δ H = 498 KJ mol^-1 OH(g)→ H(g) + O(g); Δ