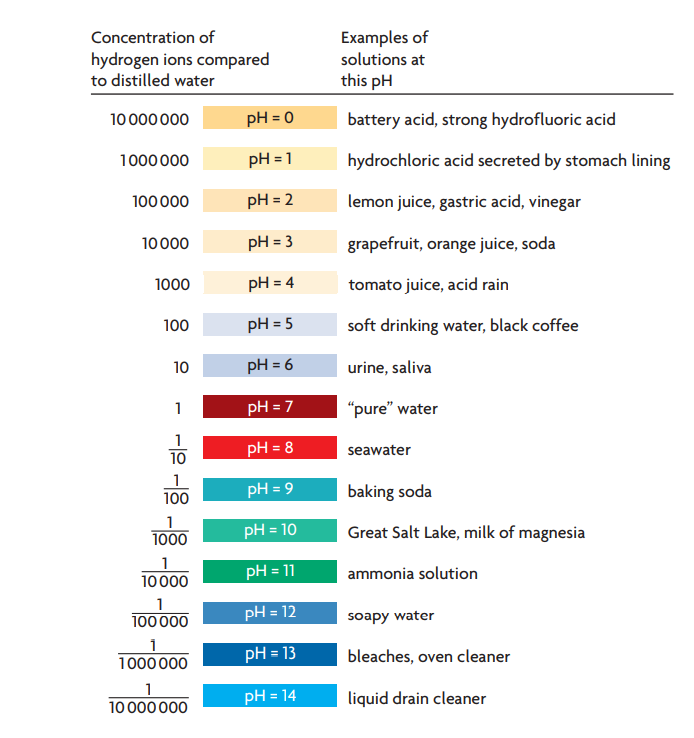
pHgraduatedscale.jpg - Concentration of Hydrogen ions compared to distilled water Examples 10 000 000 PH 0 Battery acid 1 000 000 PH 1 Hydrochloric | Course Hero
![At 80^(@)C distilled water has [H(3)O^(+)] concentration equal [OH^(-)] 1xx10^(-6) "mole"//litre. The value of K(w) at this temperature will be At 80^(@)C distilled water has [H(3)O^(+)] concentration equal [OH^(-)] 1xx10^(-6) "mole"//litre. The value of K(w) at this temperature will be](https://d10lpgp6xz60nq.cloudfront.net/web-thumb/52405125_web.png)
At 80^(@)C distilled water has [H(3)O^(+)] concentration equal [OH^(-)] 1xx10^(-6) "mole"//litre. The value of K(w) at this temperature will be

At 80^∘ C , distilled water has hydronium ion (H3O^+) concentration equal to 1 × 10^-6 mol/l . The value of Kw at this temperature would be:
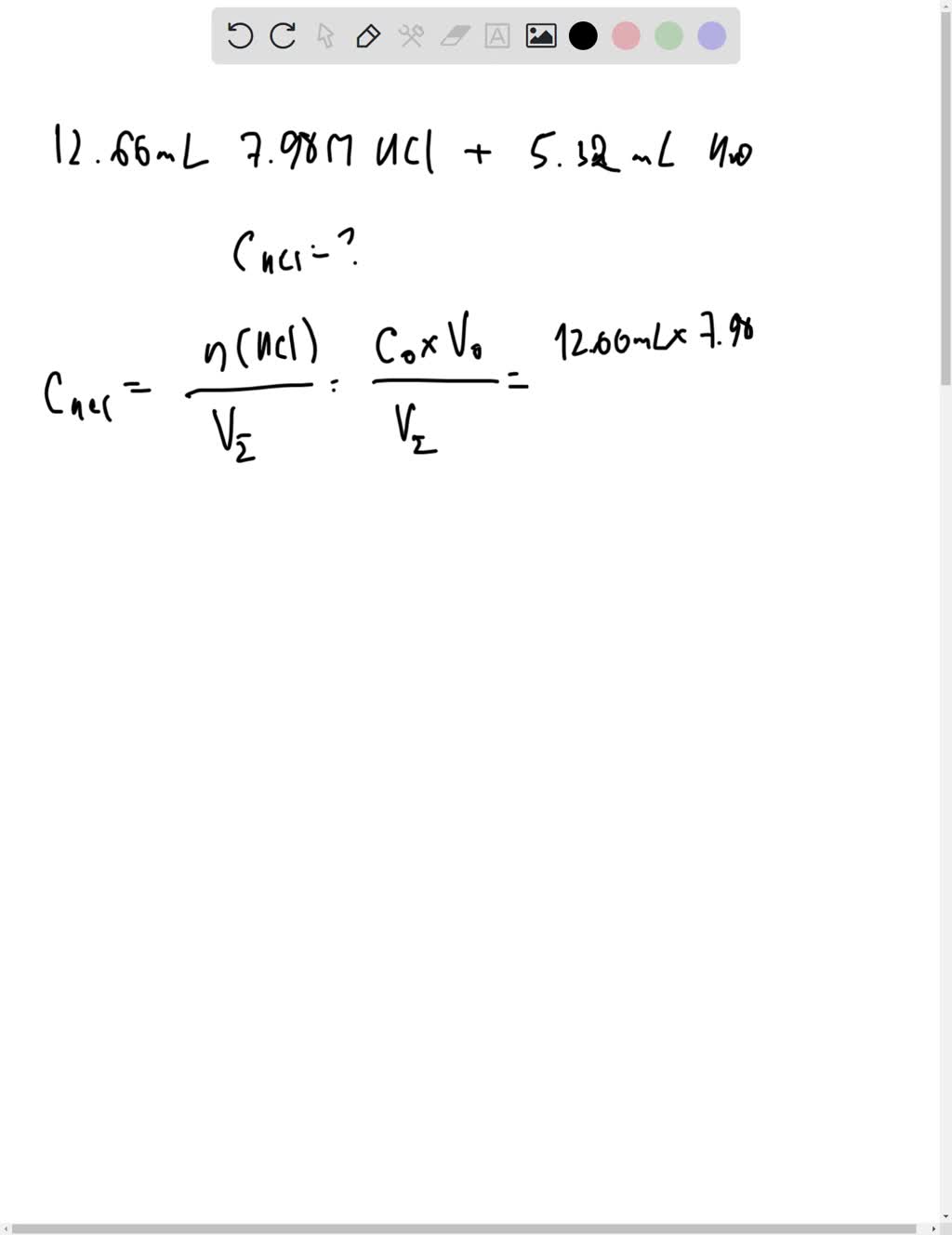
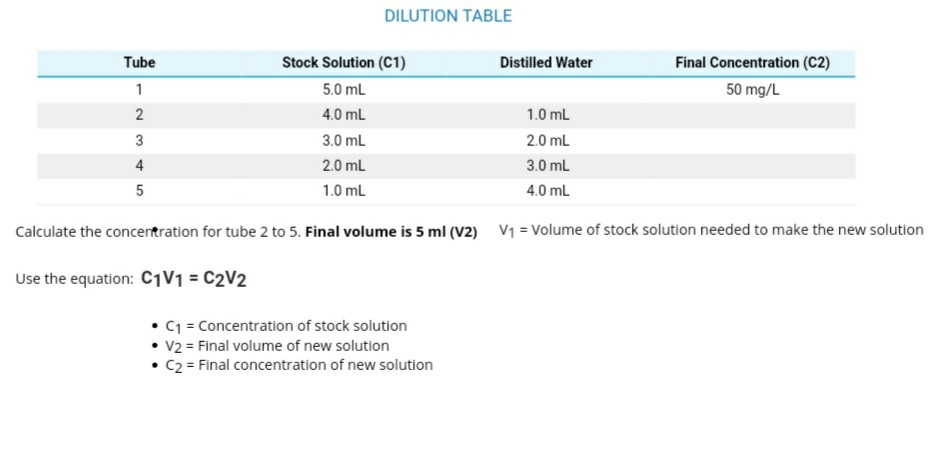
SOLVED: You will be performing many dilutions in this experiment. If John adds 12.66 mL of 7.98 M HCl to 5.32 mL of distilled water, what is the final concentration, in M, of HCl?
![At `80^(@)C` distilled water has `[H_(3)O^(+)]` concentration equal `\'^(+)O 1xx10^(-6) \"mole\"// - YouTube At `80^(@)C` distilled water has `[H_(3)O^(+)]` concentration equal `\'^(+)O 1xx10^(-6) \"mole\"// - YouTube](https://i.ytimg.com/vi/sG9XjpQwtSA/maxresdefault.jpg)
At `80^(@)C` distilled water has `[H_(3)O^(+)]` concentration equal `\'^(+)O 1xx10^(-6) \"mole\"// - YouTube

Water moved into the egg in distilled water because the higher concentration of water was outside the cell so it diffused inside t… | Glassware, Osmosis, Corn syrup
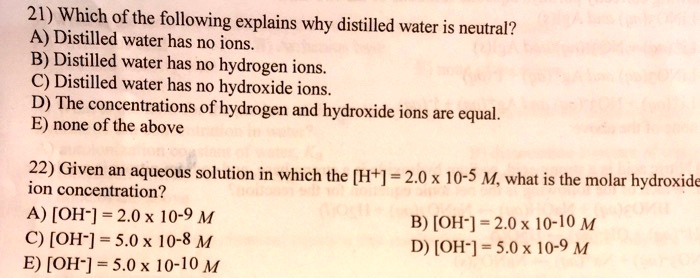
SOLVED: 21) Which of the following explains why distilled water is neutral? A) Distilled water has no ions B) Distilled water has no hydrogen ions C) Distilled water has no hydroxide ions







:max_bytes(150000):strip_icc()/water_annotated-4ab4020558324528a40132c0d842754b.jpg)