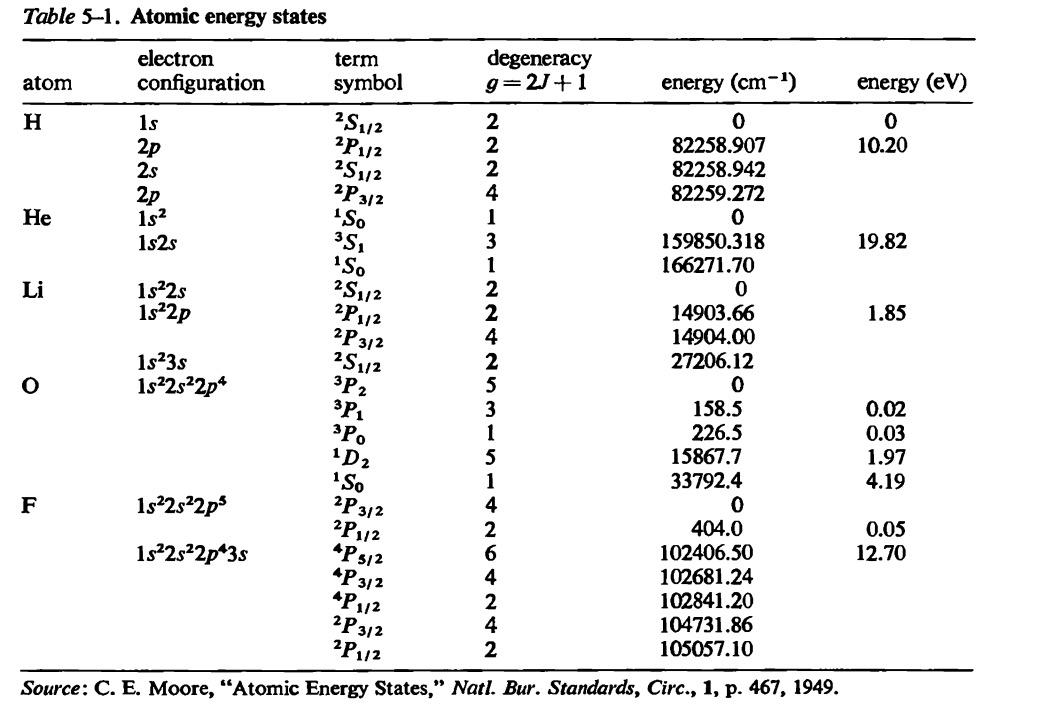
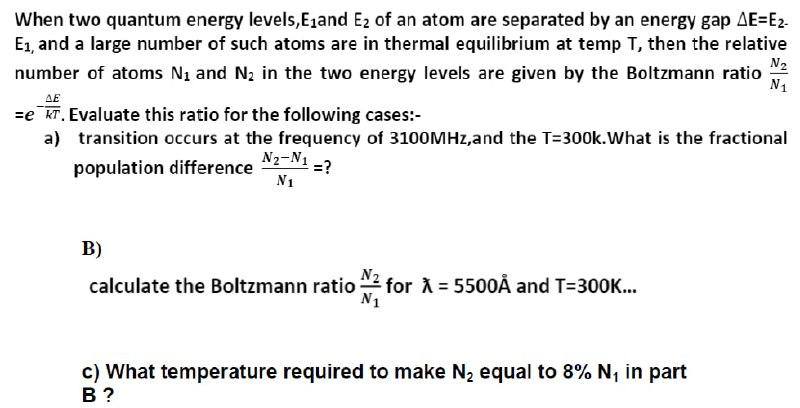
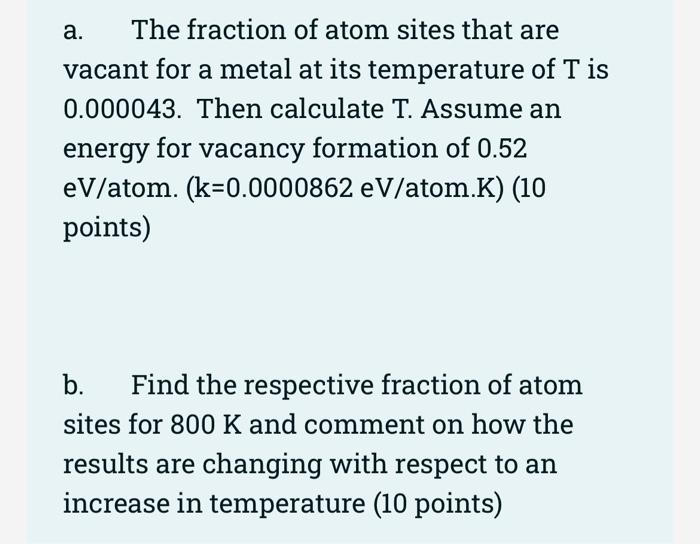
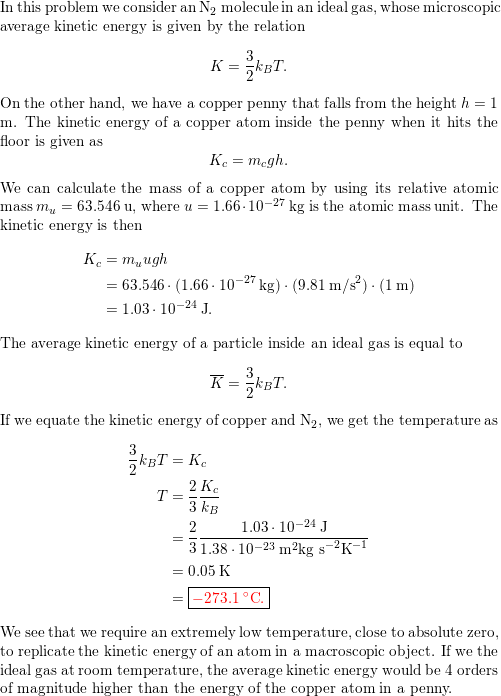Estimate the average thermal energy of a helium atom at(i) room temperature (27 ^∘C ),(ii) the temperature on the surface of the Sun (6000 K),(iii) the temperature of 10 million kelvin (the

SOLVED:Calculate the fraction of atom sites that are vacant for copper at its melting temperature of 1084^∘ C(1357 K) . Assume an energy for vacancy formation of 0.90 eV / atom.

Thermal Energy Equation & Examples | How to Calculate Thermal Energy - Video & Lesson Transcript | Study.com

Thermal Energy Equation & Examples | How to Calculate Thermal Energy - Video & Lesson Transcript | Study.com
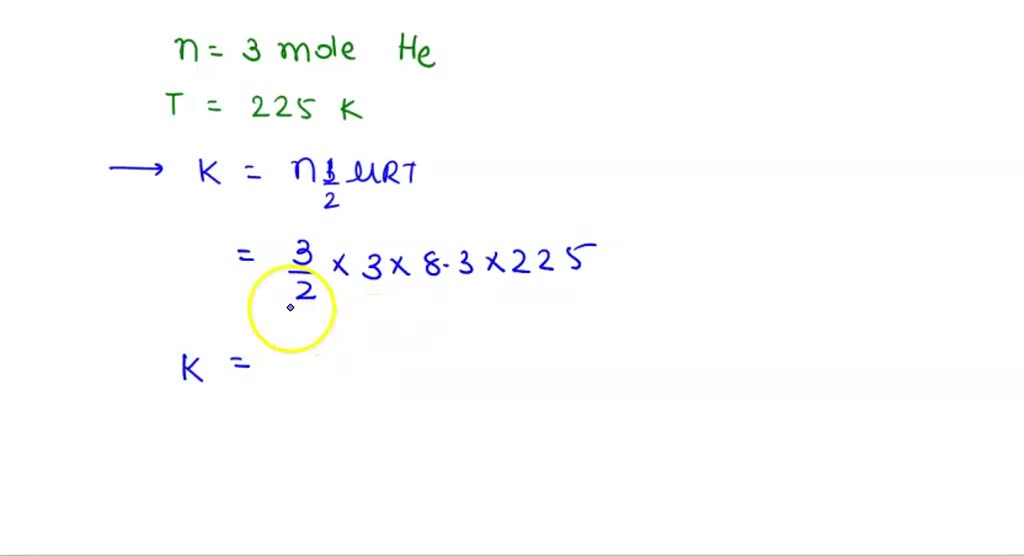
SOLVED: Five moles of a helium gas are at a temperature of 245 K. Calculate the average kinetic energy per atom, the root-mean-square (rms) speed of atoms in the gas, and the
If temperature is the kinetic energy of atoms, how can metal solids have any temperature if the atoms can't move? - Quora
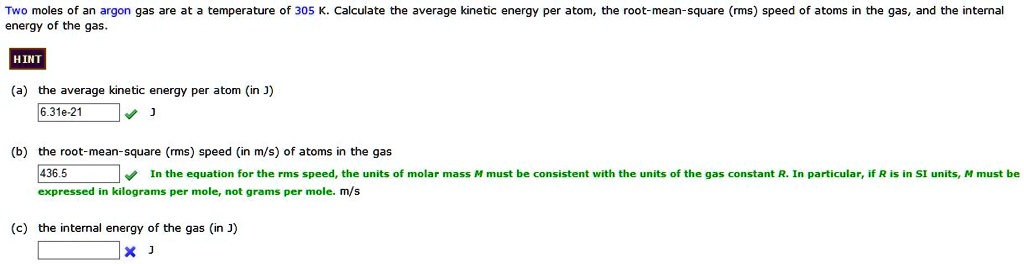
SOLVED: Two moles an argon gas are energy of the gas temperature of 305 K. Calculate the average kinetic energy per atom Toot mean square (rms) speed atoms in the gas, and

Question Video: Finding the Specific Heat Capacity of a Substance given the Change in Its Temperature and Internal Energy | Nagwa

SOLVED: Calculate the fraction of atom sites that are vacant for lead at its melting temperature of 327*C. Assume an energy for vacancy formation of 0.52 eVlatom: When, Boltznann constant K =

SOLVED:In Part IV you'll learn to calculate that 1 mole (6.02 ×10^23 atoms) of helium atoms in the gas phase has 3700 J of microscopic kinetic energy at room temperature. If we