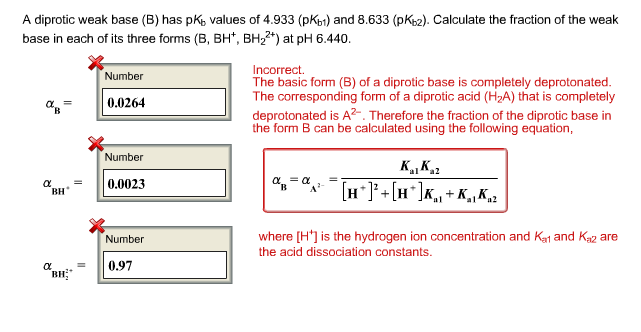
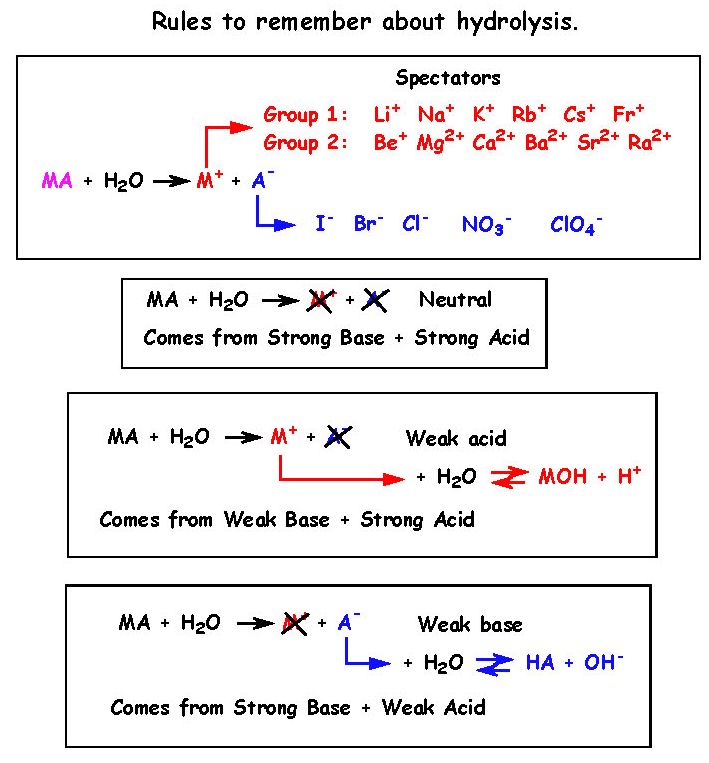
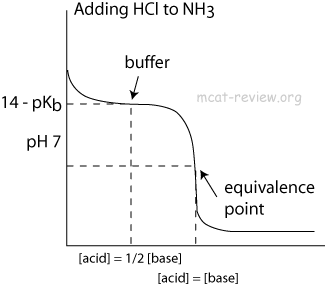
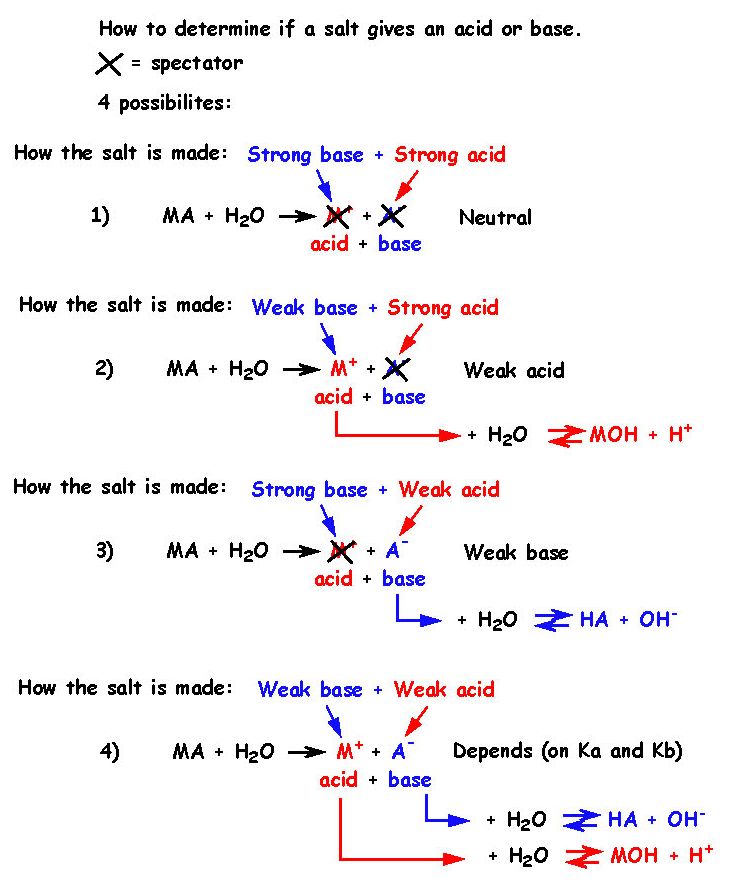
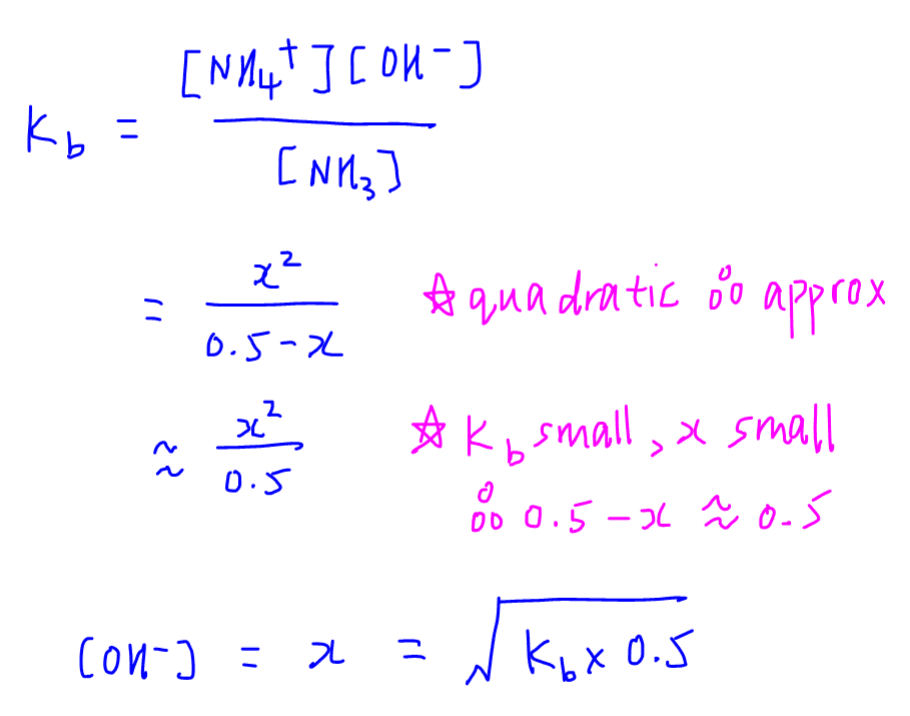The pKa of a weak acid, HA is 4.80. The pKb of a weak base BOH is 4.78. The PH of an aqueous solution of the corresponding salt, BA will be
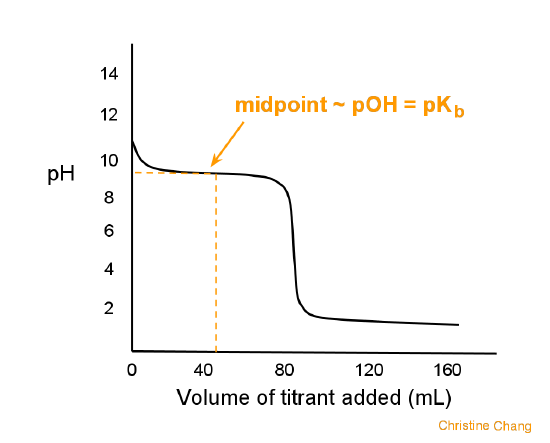
![OneClass: A weak base (B) has a pKb value of 5.77. a) At what pH is [BH ] = [B]? b) What is the predo... OneClass: A weak base (B) has a pKb value of 5.77. a) At what pH is [BH ] = [B]? b) What is the predo...](https://prealliance-textbook-qa.oneclass.com/qa_images/homework_help/question/qa_images/119/11951925.png)
OneClass: A weak base (B) has a pKb value of 5.77. a) At what pH is [BH ] = [B]? b) What is the predo...
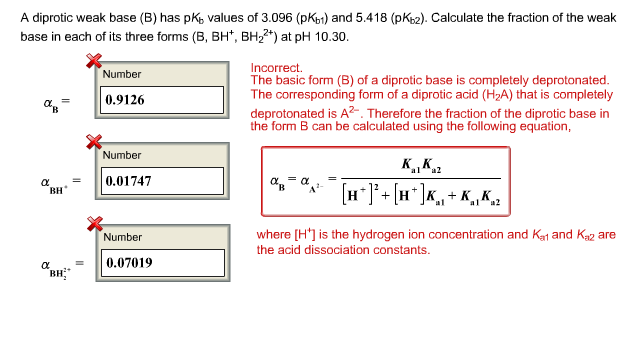
![SOLVED: Usefulequations: pH pKa + log ([conjV[WA]) where WA = weak acid pOH = pKb + log ([conjV[WB]) where WB weak base pH = -log1o[HgOt]; [H3Ot] = 10- pH pOH = log10[OH-]; [OH:] = SOLVED: Usefulequations: pH pKa + log ([conjV[WA]) where WA = weak acid pOH = pKb + log ([conjV[WB]) where WB weak base pH = -log1o[HgOt]; [H3Ot] = 10- pH pOH = log10[OH-]; [OH:] =](https://cdn.numerade.com/ask_images/614c9971ddeb4795b21a29f9ef72aa78.jpg)
SOLVED: Usefulequations: pH pKa + log ([conjV[WA]) where WA = weak acid pOH = pKb + log ([conjV[WB]) where WB weak base pH = -log1o[HgOt]; [H3Ot] = 10- pH pOH = log10[OH-]; [OH:] =
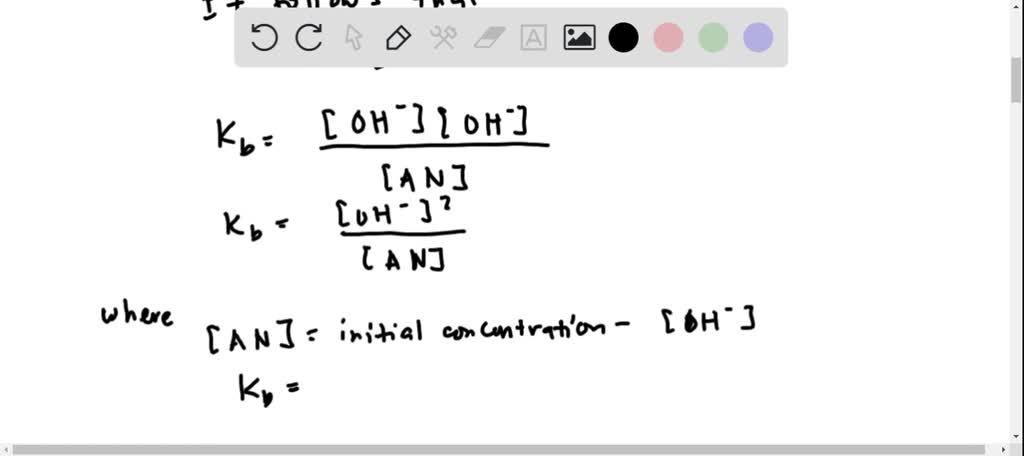
SOLVED: Amphetamine (C9H13N) is a weak base with a pKb of 4.2. Calculate the pH of a solution containing an amphetamine concentration of 225 mg>L.
The pK a of a weak acid (HA) and pKb of weak base (BOH) are 3.2 and 3.4, respectively. The pH of their salt (AB) solution is - Sarthaks eConnect | Largest Online Education Community