Consider the flasks in the following diagrams: Assuming the connecting tube has negligible volume, predict what each diagram will look like once the stopcock between the two flasks is opened. Calculate the
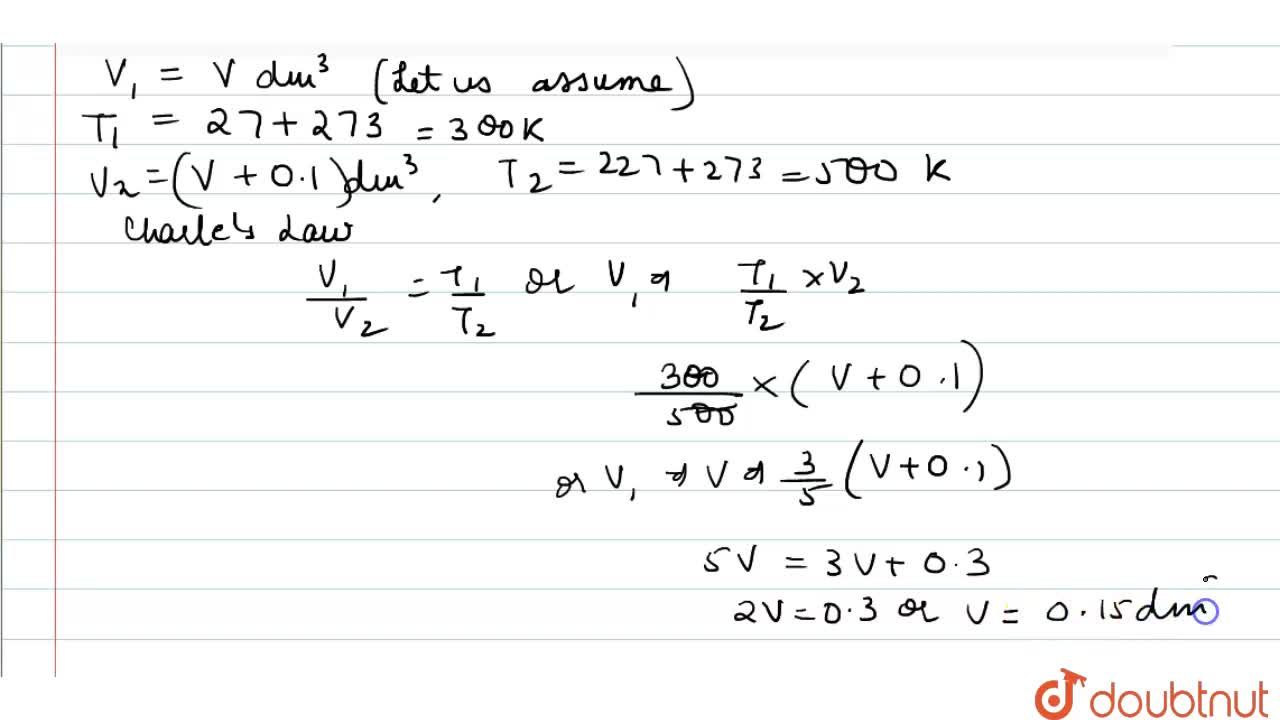
A flask was heated from 27^(@)C to 227^(@)C at constant pressure. Calculate the volume of the flask if 0.1 dm^(3) of air measured at 27^(@)C was expelled from the flask.

After completing this lesson you should be able to : Balanced equations show the mole ratio(s) of reactants and products. The molar volume is the same. - ppt download

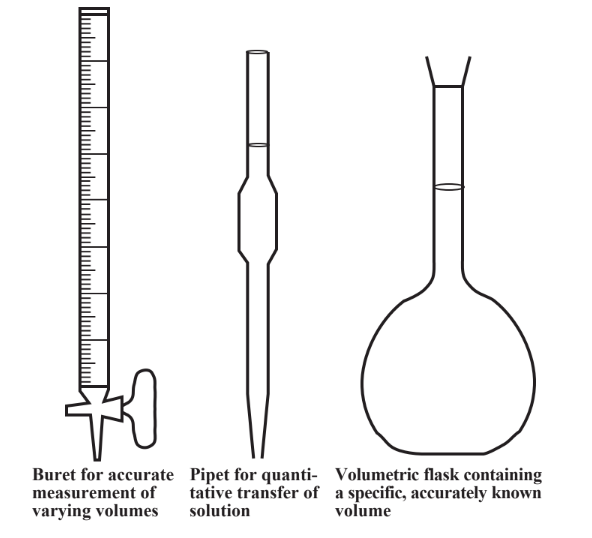
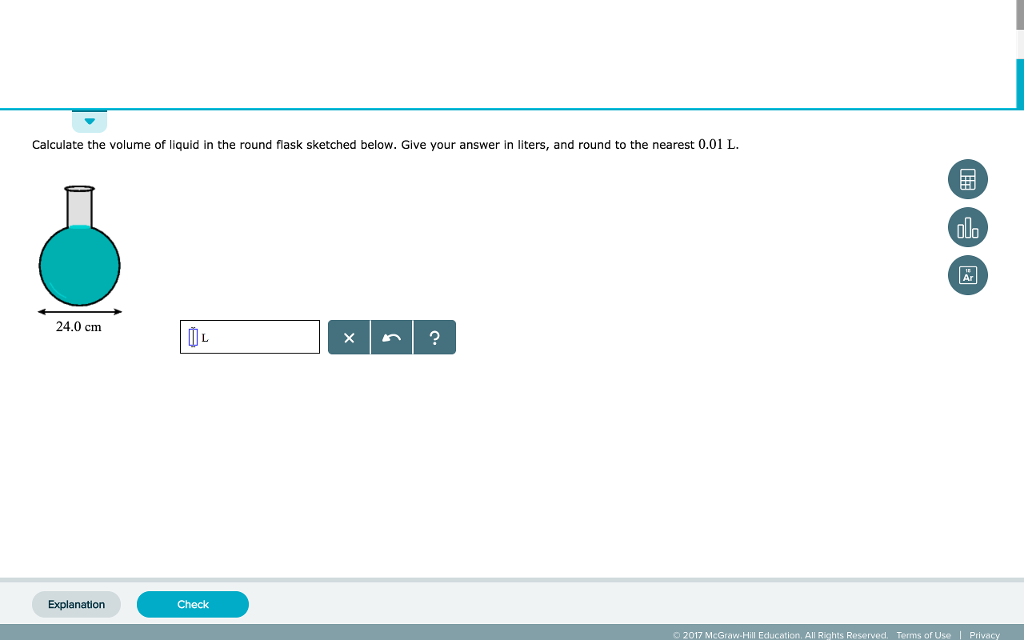
SOLVED:Finding the volume of a flask. A student obtained a clean dry glass-stoppered flask. She weighed the flask and stopper on an analytical balance and found the total mass to be 31.601
Two flasks of equal volume have been joined by narrow tube of negligible volume .Initially both flasks are at 300 Kelvin containing 0.60mol of oxygen gas at 0.5 ATM pressure . One
What is the volume of a conical flask which is 13 cm in height, with base radius of 6 cm, and an upper radius of 2 cm? - Quora

The volume of vapor in a flask can be determined by two methods: either by determining the mass of water required to just fill the flask completely or by using a graduated



![ANSWERED] A flask with a sample of gas at room temp... - Physical Chemistry ANSWERED] A flask with a sample of gas at room temp... - Physical Chemistry](https://media.kunduz.com/media/sug-question/raw/58199639-1659698160.4569945.jpeg)

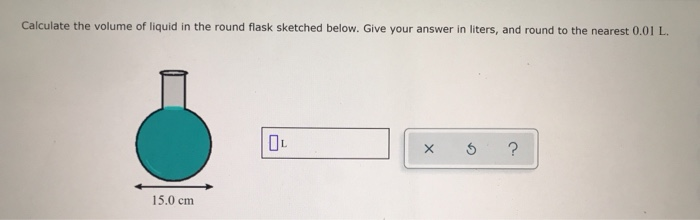
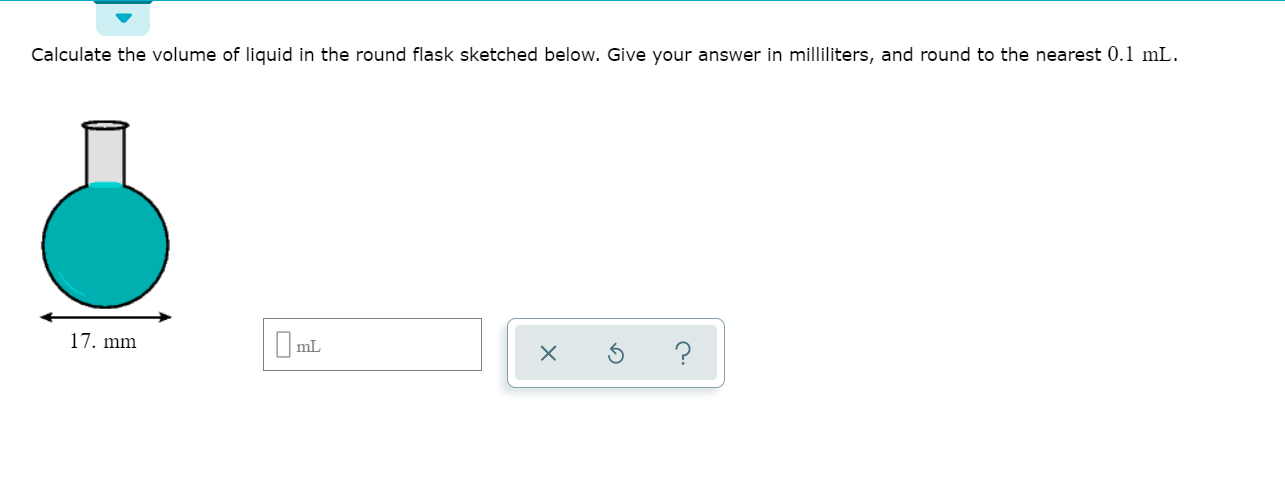
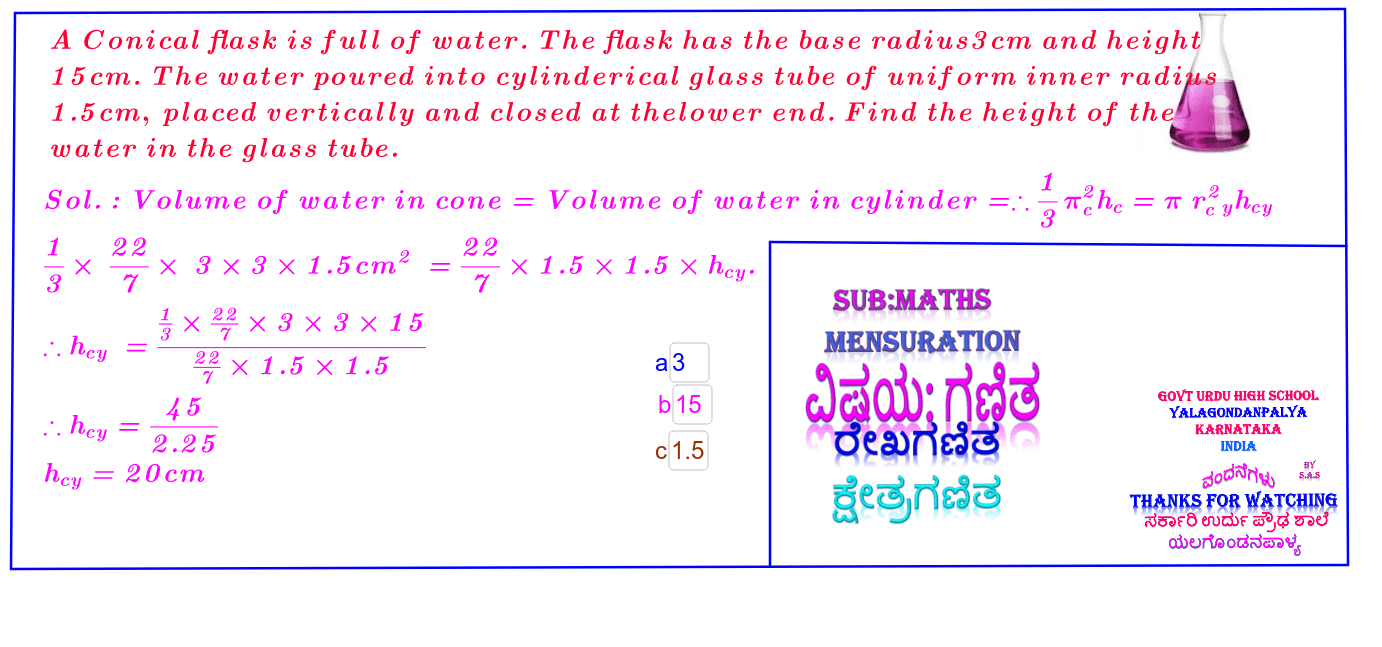
:max_bytes(150000):strip_icc()/GettyImages-493151728-c08e9b2d60bb401b8bbdd2e66de7a93b.jpg)