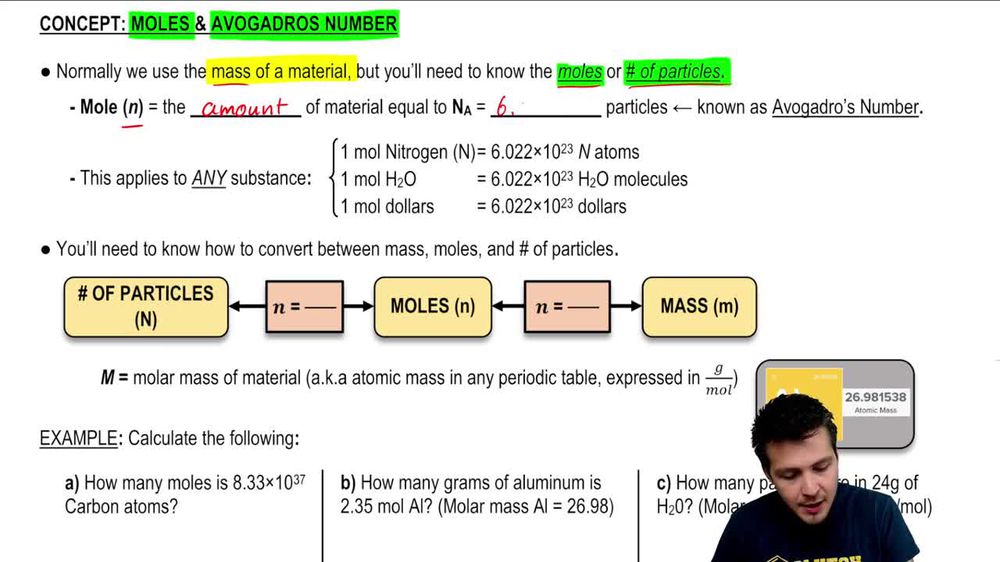
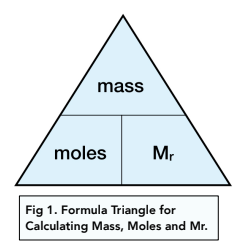
Physical Chemistry #1: Relative Mass, the Mole and Avogadro's Constant (Slides & Student Led Tasks) | Teaching Resources

Avogadro's Number - Answer Key (1).pdf - Name_ The Mole Avogadro's Number Use your scientific calculator to perform the following calculations. Use | Course Hero

Calculate the value of Avogadro number from the internuclear distance of adjacent ions in NaCl , 0.282 nm and the density of solid NaCl is 2.17 × 10^3 kg/m^3 .A unit cell

Chemistry - Relation between Mole, Avogadro number and Mass - Atoms and Molecules - Part 8 - YouTube
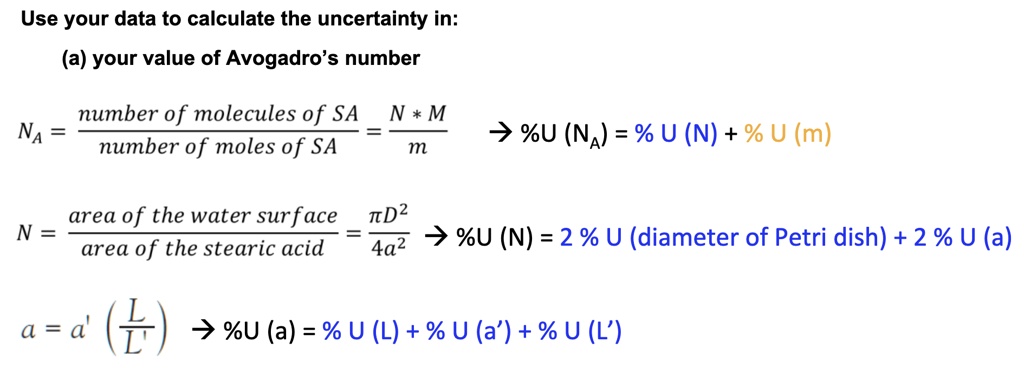
SOLVED: Use your data to calculate the uncertainty in: (a) your value of Avogadro's number number of molecules of SA N * M NA number of moles of SA m %U (NA) = %
![How to calculate number of particles| Avogadro's constant| Mole and Chemical Calculation [Online Video] – O Level Secondary Chemistry Tuition How to calculate number of particles| Avogadro's constant| Mole and Chemical Calculation [Online Video] – O Level Secondary Chemistry Tuition](https://icandochemistry942105908.files.wordpress.com/2020/12/thumb-insta-2.jpg?w=640)
How to calculate number of particles| Avogadro's constant| Mole and Chemical Calculation [Online Video] – O Level Secondary Chemistry Tuition

Section 2 - Avogadro's Number and the Mole - Part 1 | Math Tutor DVD - Online Math Help, Math Homework Help, Math Problems, Math Practice!